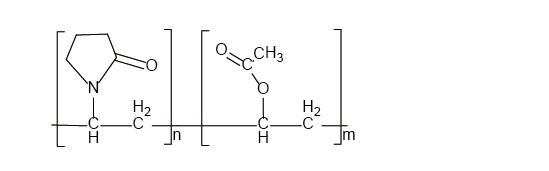
Product name KoVidone®VA64 Copovidone
USP/EP name Copovidone, Copovidonum
INCI/CTFA VP/VA copolymer 60/40
CAS NO. 25086-89-9
K value 25.2-30.8 (Copovidone 28), 27.0-33.0 (Copovidone 30)
Properties Hygroscopic capacity lower than KoVidone® K30; Soluble in water, alcohol and many other organic solvents; Glass transition temperature(Tg) lower than KoVidone® K30; Forms transparent, water removable films.
The content of vinyl acetate 35.3-41.0%
Applications
KoVidone®VA64 possesses excellent powder and film properties for broad application in the pharmaceutical field:
· Water soluble tablet binder; suitable for wet or dry granulation and direct compression processes, improves particle compressibility.
· Film-former; permeable film coating for tablet and sugar coatings to protect against splitting, decrease moisture sensitivity and provide good film adhesiveness, elasticity, and hardness.
· Porogenic agent; for use in taste-masking and component of the matrix material used in controlled-release formulation.
· Solubilizing agents; for solid dispersion processes to enhancing bioavailability and improve drug solubility. For use in both Hot Melt Extrusion and Spray Solvent Drying



